Using Predictive Intelligence to Uncover the Rare Disease Patient Journey
In this podcast interview, Dan Fisher, managing director and practice lead for IPM.ai, explains the use of artificial...
Select
In this podcast interview, Dan Fisher, managing director and practice lead for IPM.ai, explains the use of artificial...
87% of Biopharma Respondents Use AI/ML for Post-Market Activities, But Key Missed Opportunities Remain
By: Dan Fisher, Principal, IPM.ai
X‑linked myotubular myopathy (XLMTM) is a rare, life-threatening congenital...
By: Dan Fisher, Principal, IPM.ai
The Challenge
Frontotemporal dementia (FTD) is the most common form of dementia...
By: Nitin Choudhary, EVP & GM, IPM.ai
Reach diverse, trial eligible patients through the physicians they trust using...
By: Nitin Choudhary, EVP & GM, IPM.ai
Introduction
For rare and specialty oncology, low incidence rates complicate...
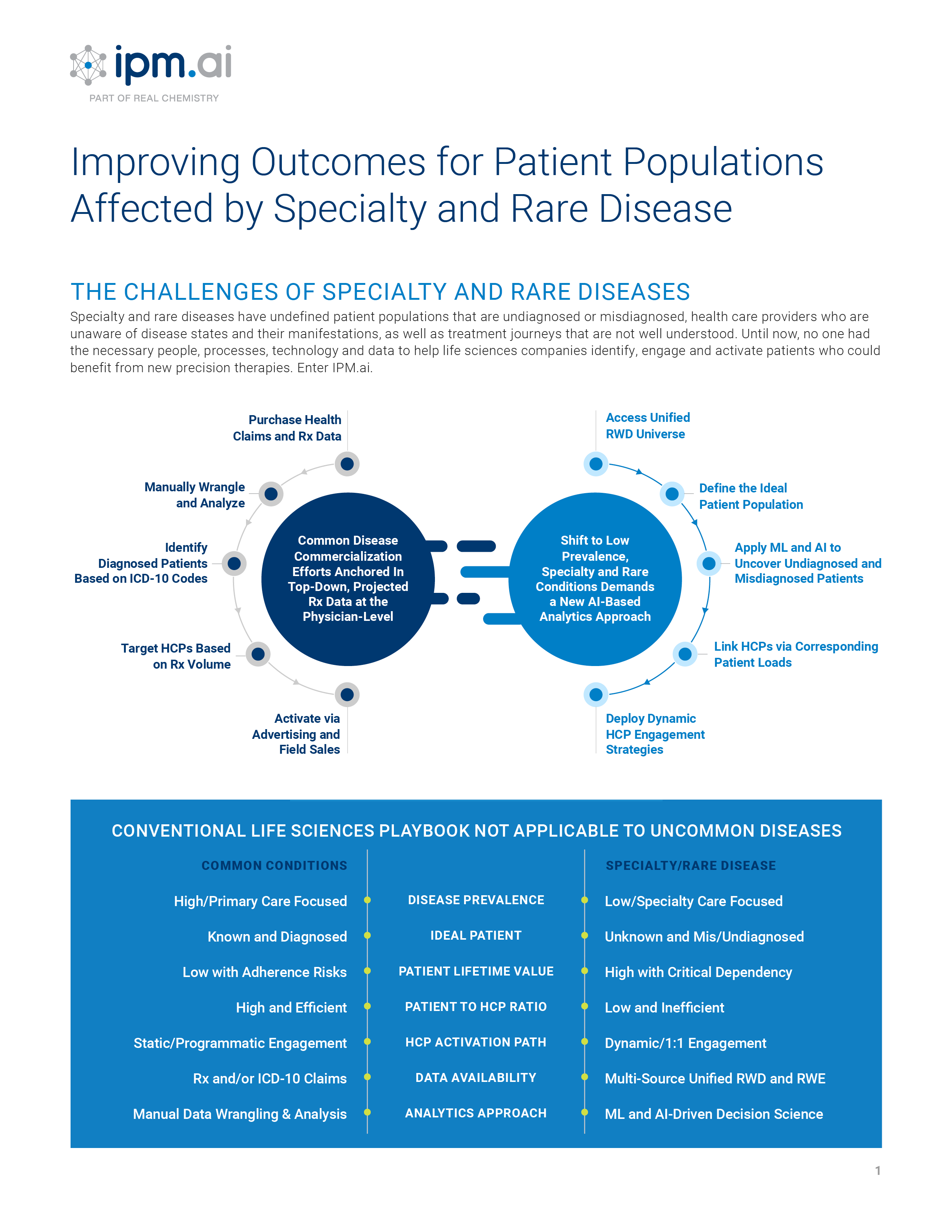
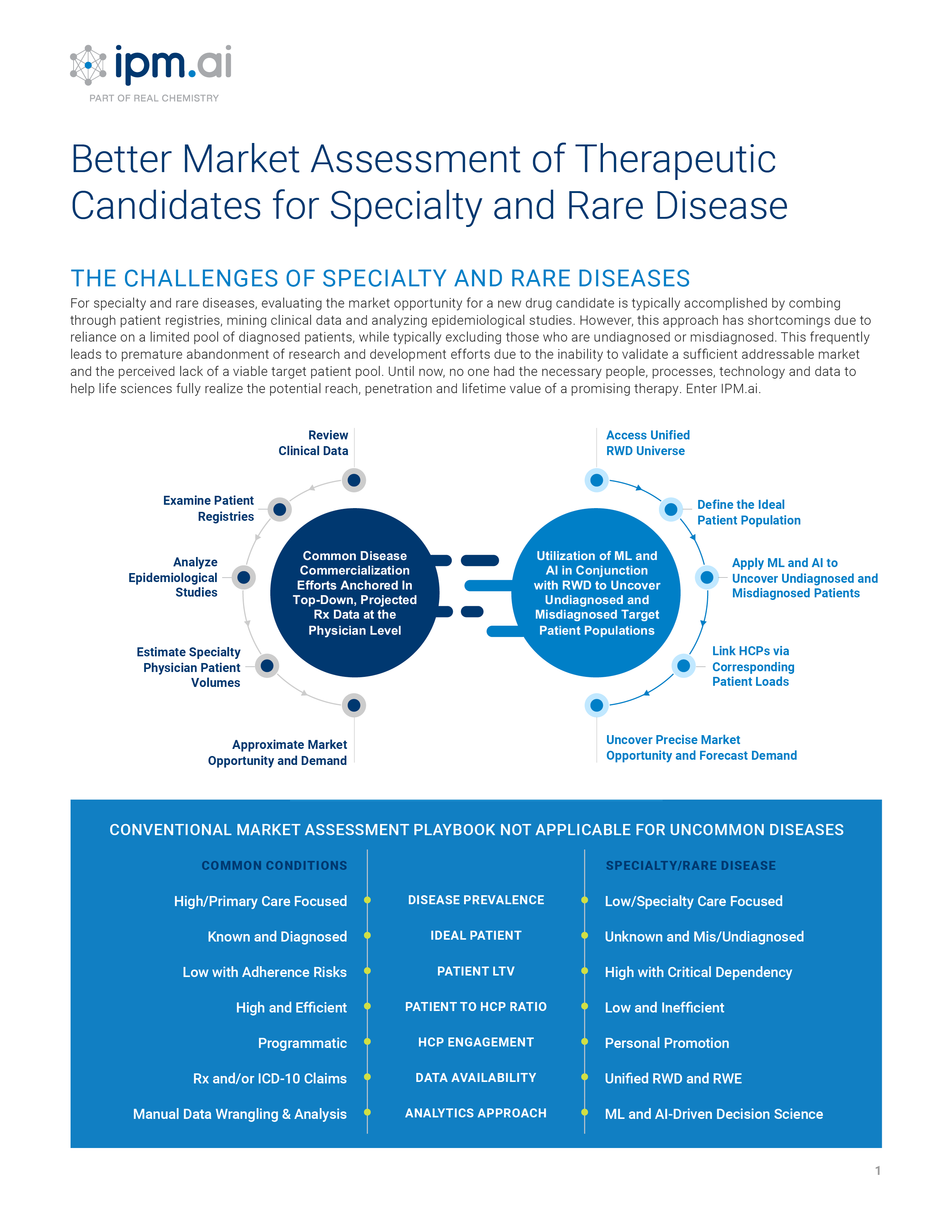
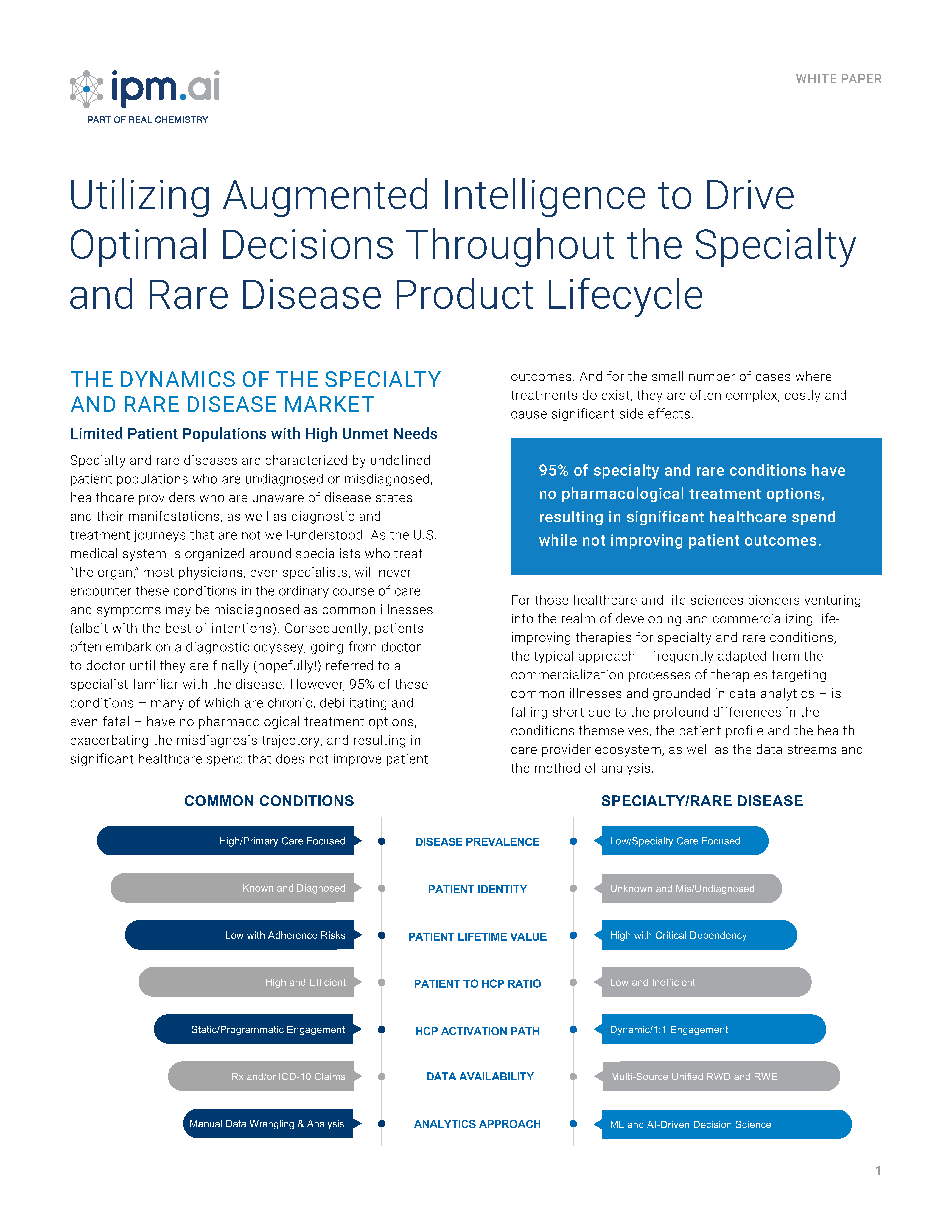
Classic approaches for patient discovery, treatment journey mapping, referral network intelligence, market assessment and audience engagement all begin with the common assumption that the combination of medical and prescription claims at the patient level can be deduced and combined to create a de-identified cohort to anchor analysis. However, as the pharmaceutical landscape shifts from one dominated by primary care markets with high prevalence and a plethora of launch blueprints to draw upon, to one where diffuse specialty markets with low prevalence and a lack of analogs to anchor launch strategy, this assumption rarely holds true and creates significant commercialization challenges. This conundrum is particularly acute in rare disease, where only about 500 of 7,000 have a diagnostic code in the International Classification of Diseases (ICD).
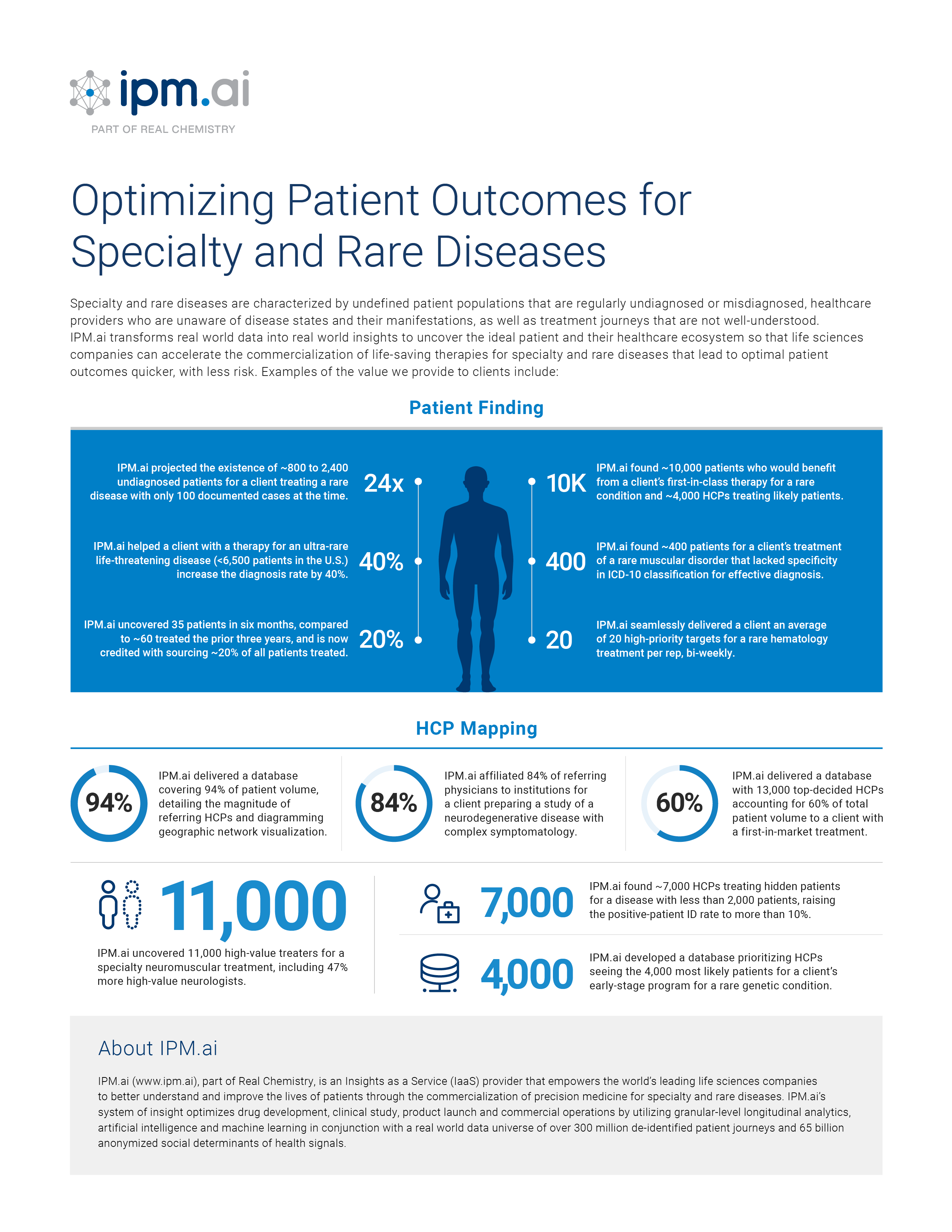
In this webinar, learn how the combination of genetic testing, the democratization of de-identified patient data, the rise of tokenization across entities in the healthcare ecosystem and machine learning can enable the promise of precision medicine. We'll also share a case study by Alnylam anchored in patient outcomes where obstacles were overcome to effectively diagnose previously undiagnosed patients.
.jpeg?width=200&name=0%20(1).jpeg) |
John GarciaVice President Global & US Business Operations
|
 |
Jonathan WoodringExecutive Vice President & General Manager
|
Jeremy Smith, Director of Business Analytics at Audentes Therapeutics and Dan Fisher, Principal Consultant at IPM.ai discuss how non-traditional real world data, combined with the power of machine learning and artificial intelligence, deepened disease understanding and shortened the diagnostic journey of X-Linked Myotubular Myopathy (XLMTM).
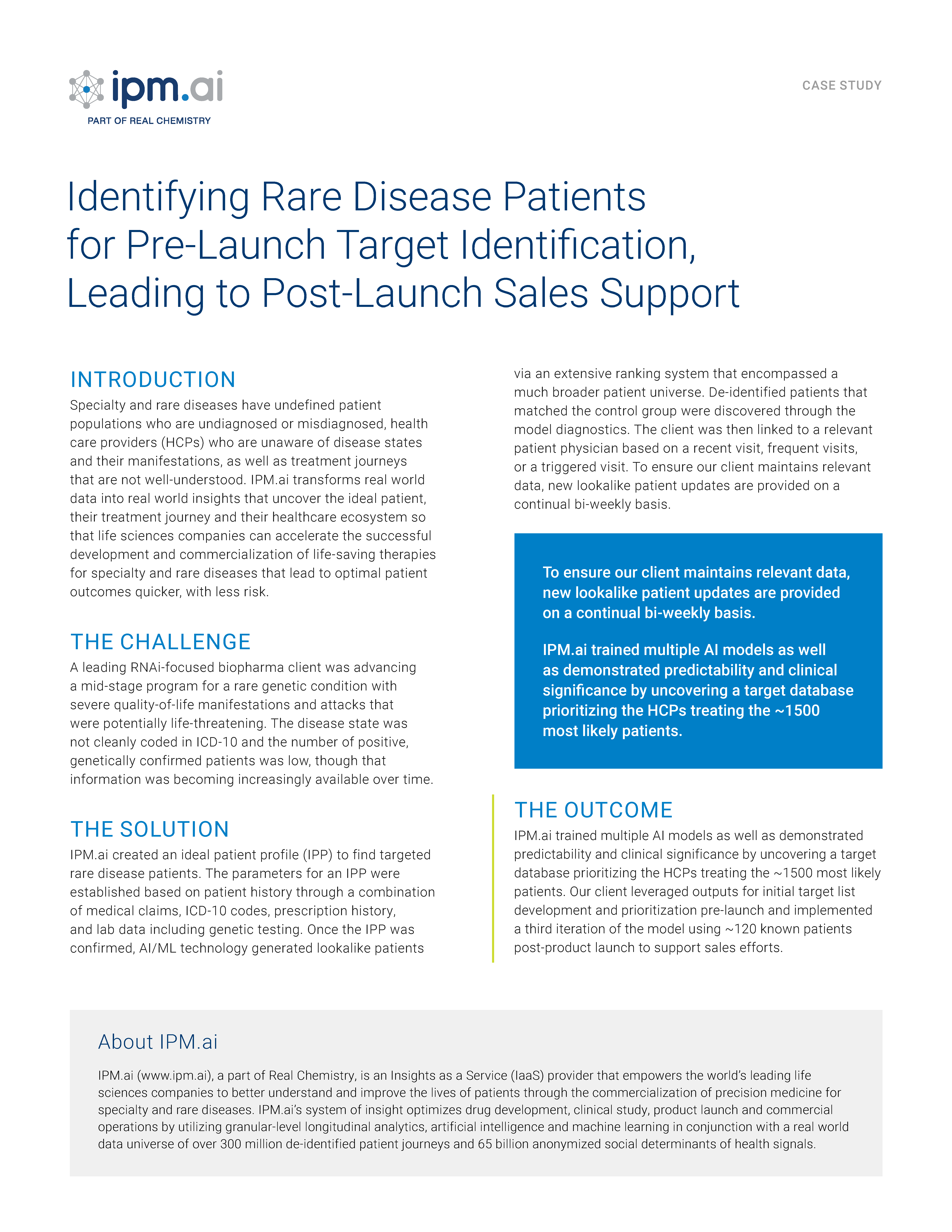
Audentes Therapeutics is developing AT132 for the treatment of XLMTM, a rare, life-threatening neuromuscular disease characterized by extreme muscle weakness, respiratory failure and high mortality. The condition, affecting about 1 in 40-50,000 newborn males, is caused by a gene mutation that leads to a lack or dysfunction of myotubularin, a protein that is needed for normal development, maturation and function of skeletal muscle cells.
The session covers how we leveraged a KOL’s home-grown patient registry in a privacy-safe fashion and unified it with a robust real world data universe to uncover the XLMTM patient journey, discover symptomatology that was not previously understood and identify undiagnosed and misdiagnosed patients to ultimately shorten the diagnostic timeline.
-Oct-07-2020-05-26-52-79-PM.jpeg?width=182&name=0%20(1)-Oct-07-2020-05-26-52-79-PM.jpeg) |
Jeremy SmithDirector of Business Analytics
|
 |
Dan FisherPrincipal Consultant
|
In this session, Biogen and IPM.ai discuss how leveraging machine learning and artificial intelligence can help improve trial recruitment. Whether it’s selecting possible location sites, expanding the list of physicians who treat the disease (vs. focusing only on a smaller subset like your target list), utilizing de-identified longitudinal real world data and cutting-edge machine learning techniques put Biogen at the forefront of clinical trial recruitment efforts.
.jpeg?width=200&name=0%20(2).jpeg) |
Adam JenkinsAssociate Director Data Science
|
 |
Nitin ChoudharyEVP & General Manager
|
Listen as we discuss the power of machine learning and artificial intelligence to engage rare disease patients who could benefit from new therapies and modalities of care, thus improving their outcomes. Together with Insmed, we discuss their business needs, illustrate our approach and relay how Insmed leveraged our partnership to facilitate earlier diagnosis and help physicians understand treatment regimes. We also review how we applied aggregated learnings from Arikayce patients against a broader medical claims database and identified patients most likely to have Refractory MAC lung disease. Finally, we reveal how Insmed leverages both personal and non-personal activation to educate physicians about disease states and the drug that could effectively treat this condition.
 |
Areejit PhukanDirector Analytics & Forecasting
|
 |
Nitin ChoudharyEVP & General Manager
|
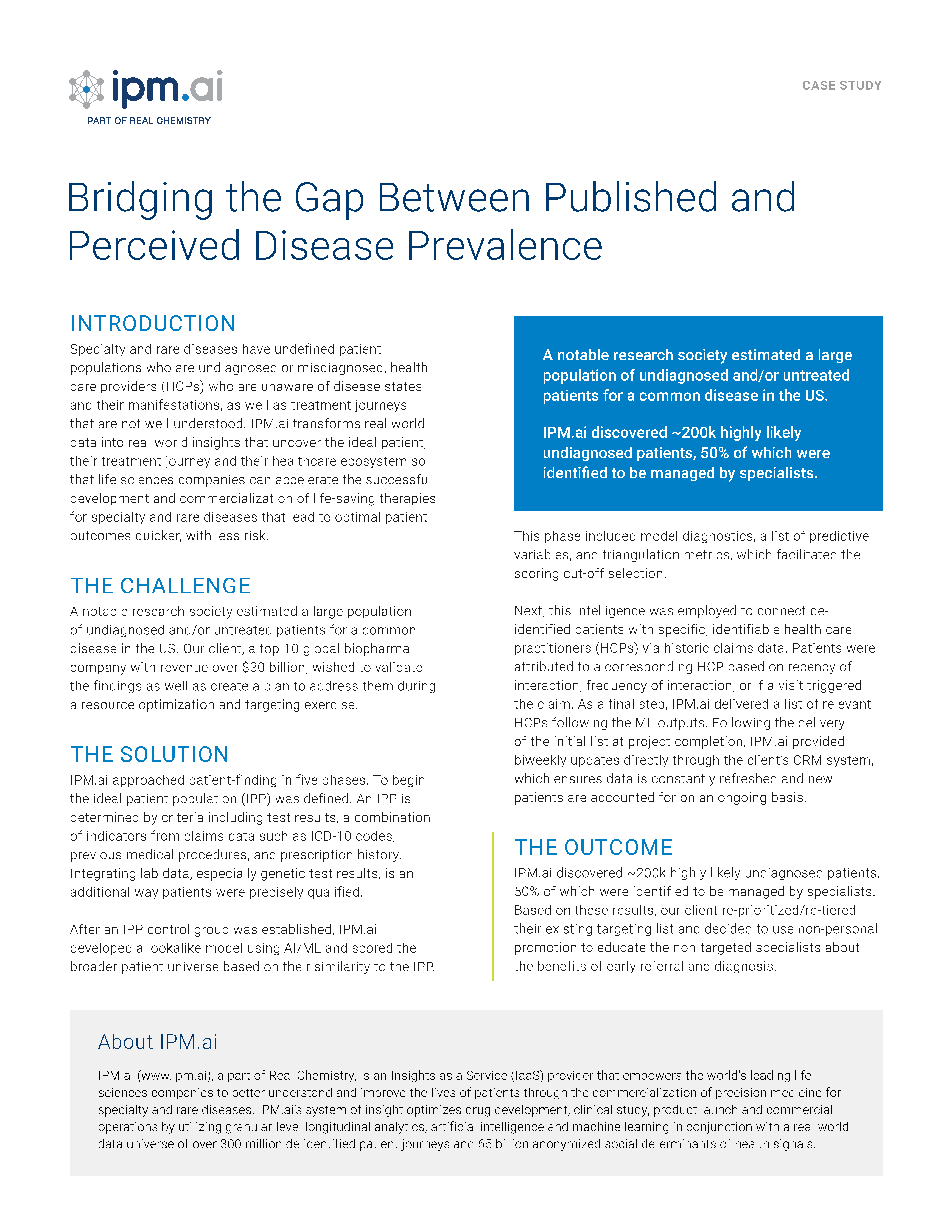
Graham Jones of IPM.ai accompanied by Keith R. McCrae, MD of the Cleveland Clinic and Jennifer Beachell, Tricia Gooljarsingh PhD and Mary Lee Tjoa of Momenta Therapeutics discussed the clinical presentation of warm autoimmune hemolytic anemia (wAIHA), a rare, Ig-G mediated disorder. There is no diagnosis code specific for wAIHA, presenting challenges in identifying wAIHA patients and assessing the clinical course of the disease. For this study, we aimed to: 1) identify a wAIHA cohort using a collection of specific diagnostic codes; 2) evaluate whether a collection of diagnostic codes could bisect severe versus non-severe wAIHA patients; 3) observe the frequency of comorbidities and anemia symptoms in severe and non-severe wAIHA groups; and 4) use a predictive model to validate clinical variables and prevalence estimates.
Methods
A de-identified, longitudinal, patient-level claims database of >300 million US patients was used for this study. wAIHA patients were identified based on a diagnosis code of “autoimmune hemolytic anemia” (AIHA) with multiple distinct events within 36 months. Patients were required to have at least 30 days use of steroids over 36 months, and utilization of a known drug regimen for treatment of an autoimmune disorder. Patients were classified as severe if claims related to transfusion or blood composition testing or if high-frequency interactions with a hematologist were observed in the 36-month period. Codes for comorbidities, treatments, and procedures were grouped and analyzed within the most recent 12 months for each patient. Prevalence was estimated using ML/AI lookalike modeling, using the known wAIHA patients as the positive training class.
Results
A cohort of 1,548 wAIHA patients was identified. Median patient age was >65 years, and patients were evenly distributed by gender. Patients showed evidence of anemia, anemia symptomology (such as shortness of breath, cough, and fatigue), and wAIHA-specific testing and treatments. The rate of disease-relevant claims was disproportionately higher in the severe cohort versus the non-severe cohort; over the 12-month study period, variances ranged from 61% higher (for anemia-based comorbidity codes) to as high as 570% higher (for anemia-based procedural codes). Primary hypertension, hyperlipidemia, gastroesophageal reflux, and evidence of chemotherapy use were also present in wAIHA patients; all of these conditions were observed more frequently in severe patients. Of interest, lupus was observed more frequently in the non-severe wAIHA cohort. Almost 44% of wAIHA claims for the full cohort were associated with Hospital/Emergency care - 48% for the severe group. AI/ML modeling predicted patients using claims variables for hemolytic anemia, other blood count abnormalities, and medical procedure claims commonly used for the diagnosis and management of wAIHA patients (such as Coombs and haptoglobin testing). The predicted population supports reported US prevalence estimates of 30,000-49,000 patients.
Conclusions
We developed and validated a method for defining wAIHA patients using de-identified claims data based on AIHA ICD-10 codes and relevant treatments. We observed that while disease manifestations are generally the same, there is an increased rate of occurrence in the severe cohort during the same 12-month period. This increase was associated with higher utilization of healthcare resources. The comorbidity of lupus was more commonly associated with less severe wAIHA patients; this may indicate that, despite comorbidities, a sharper clinical focus is placed on the wAIHA condition itself when managing more severe patients.
 |
Graham JonesPrincipal Consultant
|
Launching an orphan drug without an approved treatment? Need to estimate a forecast without really knowing the true prevalence? Does the lack of an ICD-10 code for the disease make all this even more complicated? In this webinar, IPM.ai and X4 Pharmaceuticals discuss cutting-edge methodologies via analogies and case studies that address the above conundrums common to the industry.
-2.jpeg?width=200&name=0%20(1)-2.jpeg) |
Cathy GarabedianDirector of New Product Planning
|
 |
Jonathan WoodringEVP and General Manager
|