1 min read
Using Predictive Intelligence to Uncover the Rare Disease Patient Journey
In this podcast interview, Dan Fisher, managing director and practice lead for IPM.ai, explains the use of artificial intelligence for stitching...
3 min read
![]() Nitin Choudhary
Dec 1, 2022 12:00:00 PM
Nitin Choudhary
Dec 1, 2022 12:00:00 PM

By: Nitin Choudhary, EVP & GM, IPM.ai
Reach diverse, trial eligible patients through the physicians they trust using IPM.ai’s system of insight.
The FDA recently updated its guidance on improving diversity in clinical trials, recommending that pharmaceutical companies create a “Race and Ethnicity Diversity Plan” that is shared with the agency early in clinical development. 1 This means that diversity must be accounted for even before trials start. As genetic differences can change drug outcomes, this is a necessary and long overdue step. A lack of patient diversity in trials is a worldwide problem. A study of trials involving nearly 150,000 patients in 29 countries between 1997 and 2014 showed that they were almost 90% white.2 To ensure all US-based studies going forward are representative, life science companies should leverage IPM.ai’s Trial Diversity Solution.
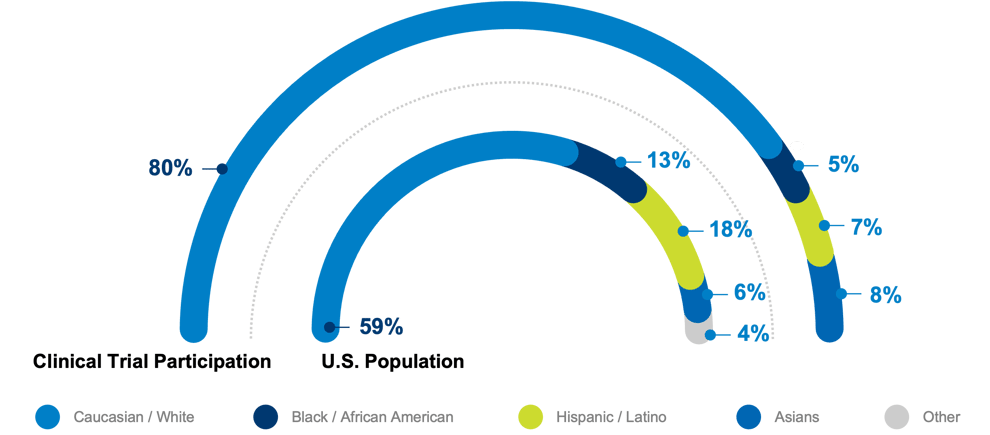
While African Americans/Blacks and Hispanics/Latinx comprise 13 percent and 18 percent of the US population, respectively, in pivotal trials submitted to the FDA supporting new drug applications from 2007-2017, African Americans/Blacks (5.4 percent) and Hispanics/Latinx (7.2 percent) were underrepresented, whereas Asians (8.6 percent) were overrepresented compared to their proportion (6.1 percent) of the US population.3-5 This misrepresentation is why the FDA is becoming more aggressive – though the latest action was a revised guidance, a mandate is unlikely far off. The industry needs a solution that accurately adds value quickly and without the demands of extensive implementation costs, time, or training, which only delays progress further.
Clearly, current tactics to recruit diverse candidates have historically fallen short. Zip-code based advertising cannot account for specific conditions; although ethnicity may be on target, this does not mean patients will qualify as trial participants. While many opt to work with a large technology platform company, these firms require time associated with implementation and training. DTC advertising of any kind is also unlikely to activate the right patients and reverse negative perceptions around trials or create access. Significant barriers include deep skepticism of clinical trials based on historical abuses, uncertainty of their value, inability to travel to sites due to a lack of transport or preventative obligations, and limited awareness of what a clinical trial entails – as well as the positive implications for the community of diversity participation.1
Though these issues aren’t going to just disappear, trial sponsors have a greater chance of activating diverse patients through the doctors they trust and rely on versus any other tactic. Patients of all ethnicities note that their physician’s recommendations have the greatest influence on their health journey and are key to enrollment. Nearly half (48%) of respondents to an Informa Pharma Intelligence survey who had participated in a clinical trial found out about it through their physician. And 81% of respondents who were not trial participants indicated they’d be more likely to enroll if their HCP referred them.6
IPM.ai can identify treating HCPs/Principal Investigators with a high potential for achieving DE&I goals through our extensive data universe, which includes more than 300 million de-identified patient health records via insurance claims with a ten-year lookback history, along with over 65 billion anonymized social determinants of health signals generated from consumer spending. These claims are refreshed weekly and always anonymized, ensuring all candidates are relevant and de-identified. As HCPs are the most effective clinical trial ambassadors, outreaching to those with greater diverse patient volume is crucial to ensuring trial inclusion.
Relying on RWD is the solution to the ongoing challenge of diverse trial recruitment – by partnering with a system of insight provider, submitting a clinical plan to the FDA early on and following through, companies can fill trials with more representative patients, faster. Once HCPs are uncovered, a company can take action – for example, using a population density map to inform where a trial site is located for easier patient access. It’s time to stop the excessive spending associated with pushing back deadlines and changing protocols due to an inability to effectively enroll diverse patients.
According to our data, approximately 14% of oncology physicians treat diverse patient populations. IPM.ai's ability to cross index diverse trial eligible patients means a more focused HCP engagement strategy — with a higher likelihood of recruitment success. Our lean, agile team can execute rapidly, enabling life science companies to move forward within weeks. Testing critical therapies in patients that reflect the US population leads to the realization of DE&I goals, eliminating diversity recruitment challenges and helping drugs get to market faster.
To get started, contact nchoudhary@realchemistry.com and begin outreach in as little as three weeks.
References:

|
Nitin ChoudharyEVP & GM, IPM.ai Nitin brings nearly 20 years of experience in life sciences analytics and consulting across specialty, oncology and rare disease at every stage of product development and commercialization. He previously served as a Principal Consultant for Symphony Health and as a Senior Engagement Manager for MarketRx (a Cognizant company). |
1 min read
In this podcast interview, Dan Fisher, managing director and practice lead for IPM.ai, explains the use of artificial intelligence for stitching...
87% of Biopharma Respondents Use AI/ML for Post-Market Activities, But Key Missed Opportunities Remain